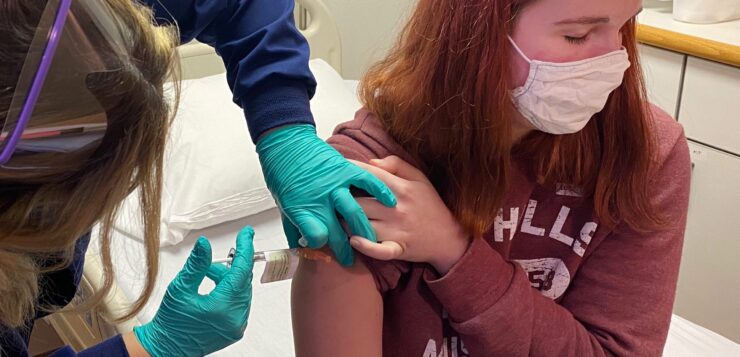
If cleared by the FDA, Moderna will become the second Covid-19 vaccine available in the U.S. for teens under 18 along with Pfizer-BioNTech’s vaccine.
By Jane Weaver
Moderna on Thursday filed for emergency use authorization from the Food and Drug Administration to use its Covid-19 vaccine in adolescents ages 12 to 17.
If cleared by the FDA, it will become the second Covid-19 vaccine available in the United States for teens under 18 along with Pfizer-BioNTech’s vaccine, which was authorized for adolescents ages 12 to 15 in May.
Moderna said in May that results from its clinical trial among children ages 12 to 17 showed its two-dose mRNA vaccine to be safe and highly effective. The trial included more than 3,700 participants.
Side effects were generally mild or moderate, the company said. Arm pain at the injection site was the most common side effect after the first dose and headache, fatigue, muscle aches and chills were most common after the second dose.
“We are encouraged that the Moderna Covid-19 vaccine was highly effective at preventing Covid-19 and SARS-CoV-2 infection in adolescents,» Moderna CEO Stéphane Bancel said in a statement Thursday.
Moderna’s vaccine for adults 18 and older has been administered in the U.S. since December. But vaccinating children has been considered key to controlling the pandemic in the U.S., the company started testing its Covid-19 vaccine in younger children ages 6 months to less than 12 years in March.
Pfizer-BioNTech also started studying their two-dose Covid-19 vaccine in kids ages 5 to 11 in March. Earlier this week, Pfizer said it was expanding its vaccine trials to include children 6 months to below the age of 5.
Moderna has already filed for authorization with Health Canada and the European Medicines Agency for the vaccine’s use in younger teens, the company said.
Follow NBC HEALTH on Twitter & Facebook.